 and Solubility KEY" width="115px" />
and Solubility KEY" width="115px" /> and Solubility KEY" width="115px" />
and Solubility KEY" width="115px" />


Directions: Follow the instructions to go through the simulation. Respond to the questions and prompts in the orange boxes.
Vocabulary: energy, gravitational potential energy, heat energy, kinetic energy, law of conservation of energy, specific heat capacity
Prior Knowledge Questions (Do these BEFORE using the Gizmo.) A battery contains stored energy in the form of chemical energy.
Some examples include phones, remote controls, and flashlights.
Most of these devices demonstrate kinetic and electrical energy
Gizmo Warm-up Energy constantly changes from one form to another, but in a closed system, the total amount of energy always remains the same. This concept is known formally as the law of conservation of energy.
TheEnergy Conversion in a System Gizmo allows you to observe the law of conservation of energy in action. In the Gizmo, a suspended cylinder has gravitational potential energy. When the cylinder is released, the gravitational potential energy is converted into kinetic energy, which causes the stirrer in the water to spin.
The temp increases, while the cylinder’s height decreases.
I think that the temp increased due to heat energy as it turned the liquid into gas
Potential energy and height
Get the Gizmo ready: ● Click Reset ( ).
Introduction: The raised cylinder in the Gizmo has gravitational potential energy (GPE) because gravity can cause the cylinder to drop. When the cylinder drops, its kinetic energy is converted into heat energy, which raises the temperature of the water.
Question: How does the cylinder’s initial height affect its gravitational potential energy?
The higher the cylinder is positioned the more heat it would create
Click Play, and record the water’s final temperature in the table below. Repeat the experiment at each cylinder height to complete the second column in the table.
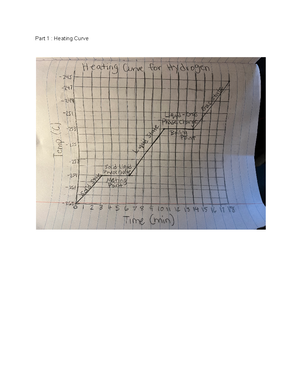
Cylinder height (m) Final temp. (°C) Change in temp. (°C) CylinderGPE (J) 100 m 26 C 1 C 4900 J 200 m 27 C 2 C 9800 J 500 m 30 C 5 C 24500 J 1,000 m 36 C 11 C 49000 J
An object’sGPE can be calculated by multiplying its height (h) by its mass (m) and acceleration due to gravity (g): GPE =mgh. On Earth,g = 9 m/s 2. Calculate the cylinder’sGPE for each of the trials you completed and fill in the last column of the table.
A. How does doubling the height of the cylinder affect itsGPE?
It will increase the number of joules being produced.
B. How does doubling the cylinder’sGPE affect the change in temperature experienced by the water?
It affects the change in temp. by doubling it.
Heat energy and temperature
Get the Gizmo ready: ● Click Reset. ● Select the GRAPH tab and choose the Generated heat option.
Question: What factors affect how much the water’s temperature changes when a given amount of heat energy is added to the water?
A. How will changing the water’s initial temperature affect how much the water’s temperature increases when the cylinder is dropped?
The cylinder after the temperature drops
B. How will changing the water’s mass affect how much the water’s temperature increases when the cylinder is dropped?
The temperature will decrease
Water’s initial temp. (°C)
Water’s final temp. (°C)
Change in temp. (°C)
Generated heat (kJ) 1 kg 0 °C 5 5 5. 1 kg 20 °C 25 5 5. 1 kg 40 °C 45 5 5. 0 kg 25 °C 36 11 11. 1 kg 25 °C 30 5 5. 1 kg 25 °C 28 3 3.
The pattern that was followed caused the amount generated to be the same each time.
A. What was the effect of the initial temperature on the temperature change of the water, and why do you think this happened?
The initial temp determined the final temp along with the mass of the water.
B. What was the effect of doubling the water mass on the temperature change, and why do you think this happened?
The final temp decrease because it got colder
Specific heat capacity can be calculated using the following equation:q =mc∆T.
In the equation,q represents the amount of heat energy gained or lost (in joules),m is the mass of the substance (in grams),c is the specific heat capacity of the substance (in J/g °C), and ∆T is the temperature change of the substance (in °C).
Click Reset. Set the water Mass to 1 kg (1,000 g). The cylinder should have a Mass of 5 kg and a Height of 500 m.
A. What is the gravitational potential energy of the cylinder? 5kg
B. If no energy is lost, how much heat energy is added to the water? 45 C
C. What is the mass of the water? 1kg
D. What is the temperature change of the water? 5 C
E. What is the specific heat of the water? (Show your work below.) 4/g
c = 24500 j / 1000 x 5 c = 4/g
F. How does the specific heat of water compare to the specific heat of iron?
The specific heat of water compares to the specific heat of iron because they both depend on the mass and temp.